The terms osteogenesis and ossification are often used synonymously to indicate the process of bone formation. Parts of the
skeleton form during the first few weeks after conception. By the end of the eighth week after conception, the skeletal pattern
is formed in cartilage and connective tissue membranes and ossification begins. Bone development continues throughout
adulthood.
Even after adult stature is attained, bone development continues for repair of fractures and for remodeling to meet changing
lifestyles. Osteoblasts, osteocytes and osteoclasts are the three cell types involved in the development, growth and remodeling
of bones. Osteoblasts are bone-forming cells, osteocytes are mature bone cells and osteoclasts break down and reabsorb bone.
There are two types of ossification: intramembranous and endochondral.
Intramembranous:
Intramembranous ossification involves the replacement of sheet-like connective tissue membranes with bony tissue. Bones
formed in this manner are called intramembranous bones. They include certain flat bones of the skull and some of the irregular
bones.
The future bones are first formed as connective tissue membranes. Osteoblasts migrate to the membranes and deposit bony
matrix around themselves. When the osteoblasts are surrounded by matrix they are called osteocytes.
Endochondral Ossification:
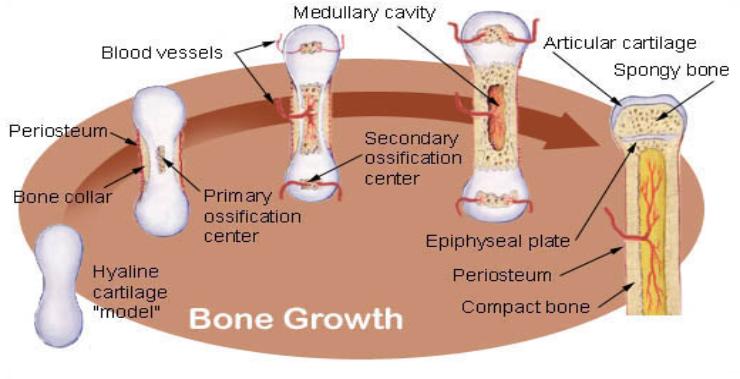
Endochondral ossification involves the replacement of hyaline cartilage with bony tissue. Most of the bones of the skeleton are
formed in this manner. These bones are called endochondral bones. In this process, the future bones are first formed as hyaline
cartilage models.
During the third month after conception, the perichondrium that surrounds the hyaline cartilage "models" becomes infiltrated
with blood vessels and osteoblasts and changes into a periosteum.
The osteoblasts form a collar of compact bone around the diaphysis.
At the same time, the cartilage in the center of the diaphysis begins to disintegrate. Osteoblasts penetrate the disintegrating
cartilage and replace it with spongy bone.
This forms a primary ossification center. Ossification continues from this center toward the ends of the bones. After spongy
bone is formed in the diaphysis, osteoclasts break down the newly formed bone to open up the medullary cavity.
The cartilage in the epiphyses continues to grow so the developing bone increases in length. Later, usually after birth,
secondary ossification centers form in the epiphyses.
Ossification in the epiphyses is similar to that in the diaphysis except that the spongy bone is retained instead of being broken
down to form a medullary cavity. When secondary ossification is complete, the hyaline cartilage is totally replaced by bone
except in two areas.
A region of hyaline cartilage remains over the surface of the epiphysis as the articular cartilage and another area of cartilage
remains between the epiphysis and diaphysis. This is the epiphyseal plate or growth region.
Bone Growth:
Bones grow in length at the epiphyseal plate by a process that is similar to endochondral ossification.
The cartilage in the region of the epiphyseal plate next to the epiphysis continues to grow by mitosis.
The chondrocytes, in the region next to the diaphysis, age and degenerate. Osteoblasts move in and ossify the matrix to form
bone.
This process continues throughout childhood and the adolescent years until the cartilage growth slows and finally stops.
When cartilage growth ceases, usually in the early twenties, the epiphyseal plate completely ossifies so that only a thin
epiphyseal line remains and the bones can no longer grow in length.
Bone growth is under the influence of growth hormone from the anterior pituitary gland and sex hormones from the ovaries
and testes.
skeleton form during the first few weeks after conception. By the end of the eighth week after conception, the skeletal pattern
is formed in cartilage and connective tissue membranes and ossification begins. Bone development continues throughout
adulthood.
Even after adult stature is attained, bone development continues for repair of fractures and for remodeling to meet changing
lifestyles. Osteoblasts, osteocytes and osteoclasts are the three cell types involved in the development, growth and remodeling
of bones. Osteoblasts are bone-forming cells, osteocytes are mature bone cells and osteoclasts break down and reabsorb bone.
There are two types of ossification: intramembranous and endochondral.
Intramembranous:
Intramembranous ossification involves the replacement of sheet-like connective tissue membranes with bony tissue. Bones
formed in this manner are called intramembranous bones. They include certain flat bones of the skull and some of the irregular
bones.
The future bones are first formed as connective tissue membranes. Osteoblasts migrate to the membranes and deposit bony
matrix around themselves. When the osteoblasts are surrounded by matrix they are called osteocytes.
Endochondral Ossification:
Endochondral ossification involves the replacement of hyaline cartilage with bony tissue. Most of the bones of the skeleton are
formed in this manner. These bones are called endochondral bones. In this process, the future bones are first formed as hyaline
cartilage models.
During the third month after conception, the perichondrium that surrounds the hyaline cartilage "models" becomes infiltrated
with blood vessels and osteoblasts and changes into a periosteum.
The osteoblasts form a collar of compact bone around the diaphysis.
At the same time, the cartilage in the center of the diaphysis begins to disintegrate. Osteoblasts penetrate the disintegrating
cartilage and replace it with spongy bone.
This forms a primary ossification center. Ossification continues from this center toward the ends of the bones. After spongy
bone is formed in the diaphysis, osteoclasts break down the newly formed bone to open up the medullary cavity.
The cartilage in the epiphyses continues to grow so the developing bone increases in length. Later, usually after birth,
secondary ossification centers form in the epiphyses.
Ossification in the epiphyses is similar to that in the diaphysis except that the spongy bone is retained instead of being broken
down to form a medullary cavity. When secondary ossification is complete, the hyaline cartilage is totally replaced by bone
except in two areas.
A region of hyaline cartilage remains over the surface of the epiphysis as the articular cartilage and another area of cartilage
remains between the epiphysis and diaphysis. This is the epiphyseal plate or growth region.
Bone Growth:
Bones grow in length at the epiphyseal plate by a process that is similar to endochondral ossification.
The cartilage in the region of the epiphyseal plate next to the epiphysis continues to grow by mitosis.
The chondrocytes, in the region next to the diaphysis, age and degenerate. Osteoblasts move in and ossify the matrix to form
bone.
This process continues throughout childhood and the adolescent years until the cartilage growth slows and finally stops.
When cartilage growth ceases, usually in the early twenties, the epiphyseal plate completely ossifies so that only a thin
epiphyseal line remains and the bones can no longer grow in length.
Bone growth is under the influence of growth hormone from the anterior pituitary gland and sex hormones from the ovaries
and testes.
Even though bones stop growing in length in early adulthood, they can continue to increase in thickness or diameter throughout
life in response to stress from increased muscle activity or to weight.
The increase in diameter is called appositional growth. Osteoblasts in the periosteum form compact bone around the external
bone surface. At the same time, osteoclasts in the endosteum break down bone on the internal bone surface, around the
medullary cavity.
These two processes together increase the diameter of the bone and, at the same time, keep the bone from becoming
excessively heavy and bulky.
life in response to stress from increased muscle activity or to weight.
The increase in diameter is called appositional growth. Osteoblasts in the periosteum form compact bone around the external
bone surface. At the same time, osteoclasts in the endosteum break down bone on the internal bone surface, around the
medullary cavity.
These two processes together increase the diameter of the bone and, at the same time, keep the bone from becoming
excessively heavy and bulky.
| Bone Development Section |
April 25–28, 2007, the 2nd Conference on Skeletal Biology and Medicine held in NYC.
This meeting, was jointly hosted at the New York Academy of Sciences and Mount Sinai School of Medicine, was
organized and chaired by Mone Zaidi, professor of endocrinology, geriatrics and adult development, and structural and chemical
biology at Mount Sinai. Cochairs were Gerard Karsenty of Columbia University and Steven Teitelbaum of the Washington
University School of Medicine.
The MHE Research would like to thank all for the use of the presentation on the MHE Research Foundation website
To view the video presentation given by Dr. Henry Kronenberg during this conference please click the link tab
This meeting, was jointly hosted at the New York Academy of Sciences and Mount Sinai School of Medicine, was
organized and chaired by Mone Zaidi, professor of endocrinology, geriatrics and adult development, and structural and chemical
biology at Mount Sinai. Cochairs were Gerard Karsenty of Columbia University and Steven Teitelbaum of the Washington
University School of Medicine.
The MHE Research would like to thank all for the use of the presentation on the MHE Research Foundation website
To view the video presentation given by Dr. Henry Kronenberg during this conference please click the link tab
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||

The MHE Research Foundation, we comply with the HONcode standard for health trust worthy information: By the Health On the Net Foundation.
Click here to verify.# HON Conduct 282463 and is the patient support link on the US Government Genetics Home Reference (http://ghr.nlm.nih.gov)
website, also linked for Patient Information on The Diseases Database a cross-referenced index of human disease, as well as the
Intute: health & life sciences a free online service providing access to the very best Web resources for education and research located in the UK
Click here to verify.# HON Conduct 282463 and is the patient support link on the US Government Genetics Home Reference (http://ghr.nlm.nih.gov)
website, also linked for Patient Information on The Diseases Database a cross-referenced index of human disease, as well as the
Intute: health & life sciences a free online service providing access to the very best Web resources for education and research located in the UK
The MHE Research Foundation is proud to be working with the EuroBoNeT consortium, a European Commission granted Network of Excellence for
studying the pathology and genetics of bone tumors.
studying the pathology and genetics of bone tumors.
| This website is regularly reviewed by members of the Scientific and Medical Advisory Board of the MHE Research Foundation. All online submission forms use (SSL AES 256 bit encryption (High); RSA 1024 bit exchange) Protocol with Privacy protection. Our goal is to make this website as safe and user friendly as possible. |


| The MHE Research Foundation is a participating member organization of the United States Bone and Joint Decade, (USBJD) & the USBJD Rare Bone Disease Patient Network |
| Written consent must be obtained to attach web pages or the files attached to this website, please email the webmaster. Email the webmaster: webmaster@mheresearchfoundation.org Materials on this website are protected by copyright Copyright © 2009 The MHE Research Foundation Disclaimer: While many find the information useful, it is in no way a substitute for professional medical care. The information provided here is for educational and informational purposes only. This website does not engage in the practice of medicine. In all cases we recommend that you consult your own physician regarding any course of treatment or medicine. This web page was updated last on 12/16/09, 4:0O pm Eastern time |
The MHE Research Foundation is proud to be an affiliate of the Society For Glycobiology






